What is energy?
This is a question which can be answered many different ways. There are easy explanations, and complicated ones; we'll try to keep it simple. Hopefully our attempt to answer this question below will give you an answer that will satisfy you, depending on what you were looking for.
Energy is sometimes defined as "the ability to do work." If an object can be put to work, then it has energy.
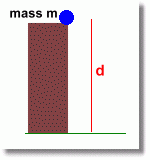
For example an object could have potential energy (due to gravity) if it were sitting up high, and could fall:
The mass m is sitting on top of the cliff, d metres above the ground. This mass clearly has potential energy ... it will do a lot of damage when it hits.
You can calculate how much energy it has using the equation E = m g d
where g is the acceleration of gravity (about 9.8 m/s2)
It may bounce, in which case the mass will have absorbed some of the kinetic energy during the collision, and is able to store it as elastic potential energy.
|
|
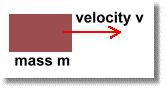
When an object is moving, it has kinetic energy, or 'energy of movement':
Clearly, the bigger mass m is, the more energy it will have as it's moving. (A baseball that hits you on the nose will do more damage than a grain of sand).
Also, the bigger the mass's velocity v, the more energy the moving mass will have. (A car hitting a wall at 100 km/h will have more damage than one hitting at 5 km/h)
You can calculate how much energy it has using the formula: E = 0.5 m v2
Find out how this applies to baseball on our 'How to Hit a Homerun' page.
|
|
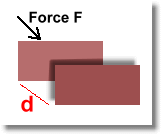
When you apply a force to an object and it moves, you're doing work, and applying energy:
The energy you apply to the object in this case is called work.
You can calculate how much energy you expended in moving the object a distance of d metres using the formula: E = F d
|
|
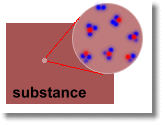
Substances always have heat energy as well:
Atoms in a substance vibrate. This represents heat energy. As long as the substance's temperature is above absolute zero, there is some heat energy.
|

|
Atoms within a substance might also be free to move around. In this case, they carry kinetic energy as well, which in this case can be thought of as heat. The hotter a substance is, the faster its atoms will be able to move around. (Or in other words, the hotter the substance is, the higher the velocity v)
|

|
Electromagnetic Energy is another common, but unusual, form of energy:
This energy can take many forms. It can be in the form of light waves, radio waves, microwaves, x-rays, or infrared rays. But one thing that is the same about all these energy forms is that the particles that make them up have no mass. They are particles that are really little bundles of 'waves', called photons, and they move at the speed of light, or 300,000,000 metres per second.
The higher the frequency of the photons, the more energy they have. Infrared photons, for example, contain more energy than radio waves, because they have a higher frequency.
You can calculate a photon's energy if you know
its frequency: E = h v
where h is a number called Planck's Constant, and v is the frequency of the waves making up the photon.
|

|
There are many forms of energy besides the ones we've already mentioned, most of which can be thought of as 'potential' until they are actually released. Here are a few.
Chemical Energy
Molecules that make up every substance are held together with energy. Sometimes when different substances combine chemically, this energy gets released. An example is wood and oxygen molecules combining, when you burn wood. This energy can be a large amount. Gasoline molecules contain a lot more energy than wood. TNT molecules contain even more. When their atoms combine with some other substance's atoms, some of the energy needed to hold them together isn't needed any more, and escapes. If it escapes fast enough, we see it as heat, light, and sound energy.
|
|
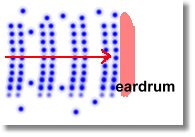
Sound Energy
This is really just a form of kinetic energy. Molecules of air are vibrated, causing them to move in wave patterns. Think of 'The Wave' that spectators at a hockey game do. When these waves (containing energy) hit your eardrum, they make it vibrate too. This vibration energy is turned into electrical energy, which your brain interprets as 'sound'.
|
|
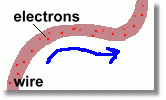
Electrical Energy
Electrons are tiny parts of an atom that can escape. They have a negative charge, and can be caused to move around if a force is applied to them. Moving electrons have energy.
You can calculate the energy flowing through an electric circuit in a certain amount of time using the formula: E = I2 R t
where I is the current, R is the resistance of the wire, and t is the time.
|
|
Mass Energy This is the most unusual form of energy, and also the one where the most energy is involved. Albert Einstein showed that mass and energy are really both forms of the same thing. That is, energy can become mass, and mass can be turned into energy. The amount of energy in a mass of m kilograms, if it were all converted to energy, can be calculated using the formula E = m c2, where c is the speed of light. Since c2 is such a huge number, this tells us that the energy in even a tiny amount of matter is enormous.
You can find out more about this on our E=mc2 page.
|
|
Here are some ways that 'mass energy' can be released:
Matter-Antimatter Reactions
Ordinary matter is made of of atoms, which contain positive protons and negative electrons. Antimatter would be made from negative protons and positive electrons. When matter meets antimatter, each and every anti-particle will combine with its corresponding ordinary particle, and they will annihilate each other ... only energy will remain.

If every particle meets an anti-particle and turns into pure energy, all of the mass gets turned into energy. You can calculate how much using E = m c2.
Antimatter can exist, ... but there can't be any left in our corner of the universe, because it would have combined with the ordinary matter that's here, and both would have disappeared.
|
Nuclear Fission
Some substances are made from really big atoms, that are unstable. They fall apart easily. When they fall apart, each of the smaller pieces is a new atom of a different substance. For example, uranium atoms fall apart easily. They might break up into two smaller atoms, like radium and lead.
When a big atom falls apart, the pieces always have less mass than the original atom. This extra mass gets turned into energy.
This process is called nuclear fission ('fission' means break apart). Since most of the mass remains behind (in several pieces), the amount of mass turned into energy is only a small fraction of the total E = m c2. In fact, it's only about 7% of that amount. But this is still a gigantic amount of energy (remember how big c2 is!)
This energy can be used to make power, or to make a bomb. You might want to find out more about how nuclear bombs work.
|
Nuclear Fusion
Other atoms can be made to combine into bigger ones. This is done by colliding them together at very high speed. When the nuclei of these two atoms combine, they form a bigger atom, but some of the mass isn't needed any more. It gets turned into energy.

Once again, there is still mass left, so the amount of energy released is less than an antimatter collision. But it is still enormous, and bigger than the energy from a fission reaction. More interestingly, this energy can be released by combining atoms of hydrogen (found in water) to make helium (a harmless gas). This process, called nuclear fusion, would allow us to get enormous amounts of energy from abundantly available material (water) with no pollution or radioactivity! Scientists are still trying to make it work; colliding the atoms requires enormous heat. But when it works, the energy released will be even greater.
Find out more about fusion here. This is the process, by the way, that happens in the sun.
|
We hope this has helped you to understand what energy is, and something about the many forms it can take. You probably already know that there are many familiar machines that are used to convert one form of energy into another. A toaster, for example, converts electrical energy into infrared (electromagnetic) energy. Your arm converts chemical energy stored in your muscles into kinetic energy when you throw a baseball. A battery converts chemical energy into electrical energy. Energy is all around you, in all its forms!
Main Page
|