|
A mass spectrograph is a device for identifying substances. It is used in laboratories to identify the chemical constituents of any sample provided, whether it be from a crime scene, a toxic substance found in a river, or an unknown chemical in a compound. In order to identify a substance, which may contain many different elements, it is first necessary to burn a small quantity of the material, and apply a charge to the particles. This gas composed of ions is collected in a container. Now the ions need to be identified. The reason for giving the atoms a charge (making them ions) is very simple. Because they are charged, they can be attracted by a magnet! In fact, if you shoot a beam of ions past a magnet, it will pull on all of them, and the less massive the ions are, the more the beam of ions will be deflected. This makes it possible to calculate the mass of an ion by measuring how much it deflects when passed through a magnetic field of known strength. This is the principle behind the operation of a mass spectrograph. Here is a diagram that shows the main parts of a mass spectrograph: 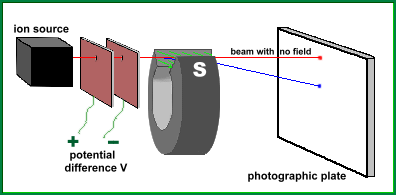 The ion source is the container holding the charged atoms of the unknown substance. When the particles escape from the box, they pass through a potential difference, which causes them to accelerate. As they pass through the magnetic field (green), the beam of ions is deflected downwards through some circular arc, which is apparent when they hit the photographic plate and leave their mark, far from where the beam would have hit had there been no magnetic field. In practice, the unknown substance will likely contain various types of ions, each with a different mass. This will result in exposures on the plate in different locations. The further down the exposure, the lighter the ion. You can calculate the mass of an ion directly, if you know the following quantities: B is the strength of the magnetic field provided by the magnet (in Newtons / amp-metre) r is the radius of curvature of the beam while in the field (in metres) V is the voltage applied between the two plates (in volts) q is the charge on an ion (1.60x10-19 coulombs if singly charged) The formula to calculate the mass of an ion is: Let's do a real example. Suppose our beam is composed of singly charged sodium ions, and is accelerated through a potential difference of 90 volts. The field strength of the magnet is 0.100 N/amp-m, and the radius of curvature of the beam is measured to be 0.066 m. Here's the calculation: This mass is very close to the mass of a sodium ion ... within 2%. Another use for the mass spectrograph, if the apparatus is made more precise by focusing the beam of ions, and making the ion's speed more uniform, is to identify the various isotopes of an element. A given material may be composed of a mixture of various isotopes of that element, and the mass spectrograph can separate them by their differing masses. In this fashion, we have learned, for example, that of all chlorine atoms found in nature, about 75% of them are Cl35, while the remainder are Cl37. (The nuclei of the isotope Cl37 have two extra neutrons) It is the mass spectrograph that has allowed scientists, over the past ninety years, to separate out and 'weigh' the isotopes of all the elements! |