|
In the last years of the 19th century, scientists were trying to discover what was inside an atom. This wasn't an easy task ... atoms were too small to see in a microscope, and too tiny to probe with any kind of common tool. They knew that material was made of atoms, but needed some way to interact with them in a way that would let them understand how they were constructed. They couldn't send a beam of other atoms into the material ... one atom will just bounce off another when the electron shells get too close. They tried sending high energy waves like gamma rays and x-rays through the material, but these just passed right through without interacting with the atoms of the material at all. What they needed was a particle smaller than an atom, and it needed to be moving fast when it penetrated the material so that it could get into the atoms. 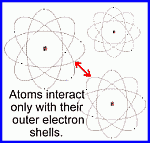 In order to understand this, you need to know about atoms.
They're mostly empty space, bounded on the outside by swarms of tiny electrons, which orbit around a tiny but very massive nucleus. Atoms interact with each other in normal circumstances by either keeping their distance from one another (due to the repulsive force of the electrons on each other), or by linking up and sharing electrons when one atom is missing some and another has extra (that's chemistry!). In order to understand this, you need to know about atoms.
They're mostly empty space, bounded on the outside by swarms of tiny electrons, which orbit around a tiny but very massive nucleus. Atoms interact with each other in normal circumstances by either keeping their distance from one another (due to the repulsive force of the electrons on each other), or by linking up and sharing electrons when one atom is missing some and another has extra (that's chemistry!).But at no time does any atom ever penetrate the electron shells of another ... the tiny nuclei remain deep inside the atom (mostly empty space, remember), untouched by any normal interaction between substances. The scientists in the late 1800's didn't know that atoms looked like this ... they only knew that they couldn't probe inside one using anything made from other atoms. So they needed something that could penetrate an atom. It had to be much smaller than an atom, and moving fast, to penetrate the electron shells and interact with what was inside. They hit upon the idea of using alpha particles. Alpha particles are tiny; they're made from just two protons and two neutrons. They can be thought of as nuclei of helium atoms, stripped of their electrons. Alpha particles are much smaller than the average atom ... in fact, they're smaller than the average nucleus, which may contain many protons and neutrons. Alpha particles can be obtained from material that is radioactive. They make up part of the 'radiation' that is emitted when unstable radioactive atoms break apart; alpha particles are some of the pieces that escape. And these move very fast! What the scientists did was to 'shine' a source of alpha particles on a piece of matter, and watch what happened to them as they penetrated the atoms of that material. Early theories of the atom held that it was a jelly-like mix of particles. If this were the case, alpha particles should be deflected a little bit as they passed through the 'jelly' of the atom ... and they should always do this. A stream of alpha particles passing through the atoms of a substance should all deflect a little bit, every time. Sort of like shooting bullets through bags of molasses. Assuming, of course, that the atom is like a bag of molasses! In fact, the result they got was astonishing. Most of the alpha particles passed through the material without interacting with atoms at all. Only a small percentage of the particles were deflected ... and some of them were deflected dramatically. In fact, some of them bounced back! It was as if they were shooting at empty bags, but the empty bags had tiny steel plates in their centres. This could only happen if there was something very massive and tiny, deep within each atom. Because atoms are mostly empty space inside, most of the alpha particles passed right through. But every now and then, one would come close to a nucleus, and be repelled. This would cause its trajectory to change. A few alpha particles had their trajectories changed a huge amount, because they happened to come very close to a nucleus. Watch this process in action here; notice that most alpha particles pass right through the material, ... but every once in a while, one will get too close to the central nucleus, and be repelled away. How long will you have to wait before one hits the nucleus dead-on and bounces backwards? In 1911, Rutherford analyzed the results of these experiments and came up with a description of the atom that makes sense. He described an atom as a cloud of electrons, orbiting a tiny but very massive nucleus in the centre. Most of the atom is empty space; only those alpha particles that happened to pass very close to a nucleus would get deflected by the Coulomb force. Physics teachers: here is a device you can build fairly easily that will replicate the scattering experiment described above. HTML, graphics & design by Bill Willis 2023 |