 Radioactivity comes in a variety of forms. We'll look at the types of radioactivity discovered by 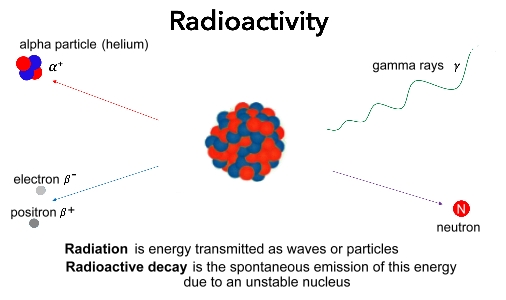 Radioactivity originates from the breakdown of the nucleus of unstable elements like uranium. Radioactivity is the radiation energy and particles that are released when that nucleus breaks down. Radioactivity originates from the breakdown of the nucleus of unstable elements like uranium. Radioactivity is the radiation energy and particles that are released when that nucleus breaks down. Some of what is released is made up of x-rays or gamma rays, photons of high-energy radiation similar to visible light or microwaves, which carry a lot of energy moving at the speed of light. Radioactive nuclei can also emit beams of fast moving physical particles. The types of particles emitted when an unstable nucleus breaks down include alpha particles, which are just nuclei of helium, neutrons, electrons, and positrons, which are the antimatter version of electrons, but with a positive charege. Sometimes the nucleus itself will break up into smaller parts, creating new elements. 
In 1911, Ernest Rutherford discovered that at the core of every atom is a nucleus. Atomic nuclei consist of electrically positive protons and electrically neutral neutrons. These are held together by the strongest known fundamental force, called the strong force. The nucleus makes up much less than .01% of the volume of the atom, but typically contains more than 99.9% of the mass. (The chemical properties of a substance are determined by the negatively charged tiny electrons swarming around the nucleus. The number of electrons usually matches the number of protons in the nucleus. We won't be discussing that here, as we're dealing strictly with nuclei). Some nuclei are unstable and may undergo radioactive decay, eventually arriving at a stable state through the emission of photons (gamma decay), emission or capture of electrons or positrons (beta decay), emission of helium nuclei (alpha decay), or a combination of these processes. We'll look at this in detail later on this page. Radioactivity can also be referred to as radioactive decay or nuclear decay. When we look at larger and larger nuclei (corresponding to elements higher and higher on the periodic table), the strong nuclear force has more and more difficulty trying to hold all the protons and neutrons together. The electric force pushing the positively charged protons in a nucleus apart is enormous, but at very short distances the strong nuclear force is even more powerful. Nevertheless, ever-larger nuclei can make it difficult for the strong force to 'corral' all the protons and neutrons. Occasionally there is some 'leakage'. This is radioactivity! While theoretically all elements can be considered unstable, most of the stable ones have a likelihood of decay that is infinitesimally small, with lifetimes longer than the age of the solar system. On the other hand, some unstable elements break down very quickly. A sample of radon, for example, can break down into polonium in days, emitting huge amounts of alpha particles. Polonium then can break down into lead and more alpha particles within a few minutes. Here is part of the 'decay chain' for uranium: 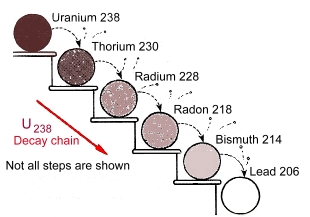 The half life of a sample of uranium-238 to turn into lead is 4.5 billion years. The chances of a particular nucleus decaying are based on probabilities. It is impossible to predict when a single nucleus will break down. Scientists describe the chances of the breakdown of nuclei in terms of half-life. Half-life is the length of time it takes for half of the radioactive atoms of a specific element to decay. For example, technetium-99 , one of the most common medical isotopes used for imaging studies, has a half-life of just 6 hours. Half of the radioactive technetium atoms will have turned into something else after six hours. The short half-life of technetium helps keep the dose to the patient low. After 24 hours, the radioactivity from the procedure will be reduced by more than 90%. A good rule of thumb is that, after seven half-lives, you will have less than one percent of the original amount of radiation being emitted. Naturally occurring uranium-238 present in the Earth’s crust has a half-life of almost 4.5 billion years. If you take a soil sample anywhere in the world, including your backyard, you will find uranium atoms that date back to when the Earth was formed. An isotope is a radioactive form of an element, one that has the same number of protons in the nucleus (and therefore the same chemical properties), but a different number of neutrons. There are stable isotopes, which do not emit radiation, and there are unstable isotopes, which do emit radiation. The latter are called radioisotopes. There are more than 3000 known radioisotopes. They are the unstable forms of elements. They emit different levels of radiation, which makes them useful in medicine, industry, agriculture, pharmaceutical sciences, industrial applications, environmental tracing and biological studies. Radioisotopes are artificially and safely produced in research reactors and accelerators. One use for radioisotopes is to manage cancer and chronic diseases using radioisotope therapy, which treats cancerous cells in a safe and effective manner. Other uses include creating better health care products by removing or neutralising chemicals, bacteria and toxins which pose a hazard. Carbon-14 is an isotope of harmless carbon, atoms of which are found in the molecules of every living thing. Carbon-14 has a half-life of 5,730 years. It is used to date materials that are organic. Find out more about how carbon-14 is used to date materials here. The radioactive waste from nuclear reactor spent fuel rods consists primarily of cesium-137 and strontium-90, but it may also include plutonium. Cesium-137 and strontium-90 have half-lives of approximately 30 years. However, plutonium has a half-life that can stretch to as long as 24,000 years. Most large elements tend to be radioactive. These large radioactive elements often undergo alpha decay, as this quickly lowers the number of protons and neutrons in the nucleus. Most nuclides found in nature are not radioactive, because all of the short-lived radioactive nuclei have already decayed, leaving a vast majority of stable nuclei. One common misconception about radioactivity is that any radioactive object is harmful to human health. This is not the case, as very small doses of radiation have not been proven to be harmful to humans. Background radiation, for example, the radiation from naturally occurring radioactive materials in the ground beneath us, is generally harmless, and can double or triple from place to place. In the natural environment, radium occurs at trace levels in virtually all rock, soil, water, plants and animals. If you live in Regina, for instance, the ground beneath your feet is twice as radioactive as it is in Winnipeg. Many products you purchase, depending on what they are made from, can be radioactive, but pose no health threats to humans. Bananas, smoke detectors, some ceramic dishware, cat litter, beer, and brazil nuts are all radioactive. However, in larger doses, radiation does have negative effects on health. When radioactive materials decay, they produce ionizing radiation. This type of radiation can strip electrons away from atoms or break chemical bonds (to make ions). This causes damage to living tissues that cannot always be repaired. Chronic exposure to high levels of radium can result in an increased incidence of bone, liver or breast cancer. As radium decays it creates a radioactive gas, radon. Radon is common in many soils and can collect in homes and other buildings. Radon is the second leading cause of lung cancer in the United States. Other effects from acute exposure to radiation appear quickly, and include burns and radiation poisoning. The symptoms of radiation poisoning include nausea, weakness, hair loss, and diminished organ function, and this radiation sickness can result in death if the dose is high enough. See our page about Marie Curie. Types of Radiation 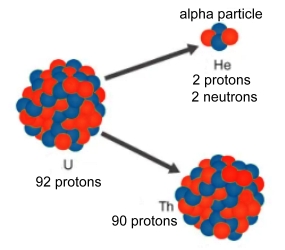 Alpha Radiation
Alpha RadiationAlpha radiation occurs when an atom undergoes radioactive decay, giving off a particle, called an alpha particle, consisting of two protons and two neutrons (essentially the nucleus of a helium atom), changing the originating atom to one of an element with an atomic number 2 less and atomic weight 4 less than it started with. In the example at the right, uranium emits an alpha particle, and the nucleus left behind is now thorium. Due to their charge and mass, alpha particles interact strongly with matter, and only travel a few centimeters in air. Alpha particles are unable to penetrate your outer layer of dead skin cells, but are capable, if an alpha emitting substance is ingested in food or air, of causing serious cell damage from inside. Alexander Litvinenko is a famous example; he was poisoned by polonium-210, an alpha particle emitter, in his tea. Uranium decays by alpha particles. External exposure to uranium is therefore not as dangerous as exposure to other radioactive elements because the skin will block the alpha particles. Ingestion of high concentrations of uranium, however, can cause health effects, such as cancer of the bone or liver. Inhaling large concentrations of uranium can cause lung cancer from the exposure to alpha particles. Polonium-210 has a half-life of 138 days, and emits alpha particles, which carry high amounts of energy that can damage or destroy genetic material in cells inside the body.  Beta Radiation
Beta RadiationBeta radiation takes the form of either an electron or a positron (a particle with the size and mass of an electron, but with a positive charge) being emitted from an atom. Due to the tiny mass of these particles, they are able to travel further in air, up to a few meters, but can be stopped by a thick piece of plastic or even a stack of paper. They can penetrate skin a few centimeters, posing somewhat of an external health risk. However, the main threat is still primarily from internal emission from ingested material.  Gamma Radiation
Gamma RadiationGamma radiation, unlike alpha or beta, does not consist of any particles. Instead it is made up of photons of electromagnetic energy called gamma rays, being emitted from an unstable nucleus. Having no mass or charge, gamma radiation can travel much farther through air than alpha or beta radiation, losing an average of half its energy for every 150 metres. Gamma waves can be stopped by a thick or dense enough layer of material, with high atomic number materials such as lead or depleted uranium being the most effective form of shielding. 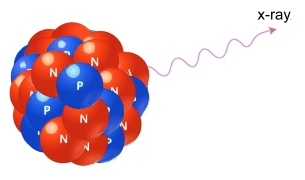 X-ray Radiation
X-ray RadiationX-rays are electromagnetic energy similar to gamma radiation, with the primary difference being that they originate from the electron cloud surrounding the nucleus. This is caused by energy changes in an electron, such as moving from a higher energy level to a lower one, causing the excess energy to be released. X-rays are longer-wavelength and usually lower energy than gamma radiation.  Neutron Radiation
Neutron RadiationNeutron radiation consists of a free neutron, usually emitted as a result of spontaneous or induced nuclear fission, where an atom of a radioactive substance breaks down. Able to travel hundreds or even thousands of metres in air, neutrons can nevertheless be effectively stopped if blocked by a hydrogen-rich material, such as concrete or water. Not typically able to ionize an atom directly due to their lack of a charge, neutrons most commonly are indirectly ionizing, in that they are absorbed into a stable atom, thereby making it unstable and more likely to emit ionizing radiation of another type. Neutrons are, in fact, the only type of radiation that is able to turn other materials radioactive. |